TAQ1
|
Biomolecule |
Type of biomolecule: (Carbohydrate, Protein, Lipid) or nucleic acid |
Role(s) within an Organism |
|
Antibody |
Protein |
Immunoglobulins, also called antibodies, are responsible for detecting and eliminating such pathogens as bacteria and viruses in a human body. They attach themselves to antigens in order to target them for destruction by the immune cells (Lyon et al. 2021). |
|
Testosterone |
Lipid (Steroid Hormone) |
Testosterone is sex hormone that is known to be present in males and sometimes in females. It helps in the variation of the second sexual characteristics, muscle and bone strength, and reproductive sperm production. To a certain extent, thoughts, feelings and communication are affected by videotaped programs with regard to behavior and mood. |
|
Triglycerides (situated within adipose tissue) |
Lipid |
Triglyceride is the other type of lipids that occur mainly in the adipose tissue where they store energy in the form of fats. It insulates and protects organs and stores energy in the case of meat during periods of low food consumption or increased energy requirements. |
|
Transfer RNA |
Nucleic Acid |
Protein synthesis is the process in which tRNA plays a major part. It delivers individual aminoacyl – tRNAs to the ribosome, correctly charging them where the new amino acid involves incorporation into the polypeptide chain according to mRNA codon. |
|
Albumin in plasma |
Protein |
Albumin is also one of the most abundant proteins found in the plasma. It regulates osmotic pressure, transports hormones, drugs as well as fatty acids and acts as a carrier for molecules in circulation (Cook et al. 2023). |
|
Glycogen |
Carbohydrate |
Glycogen is a complex carbohydrate, which is formed from the glucose molecule and functions as a storage form of glucose in animals. It is mainly deposited in the liver and muscles and can easily be utilised for energy when required. |
|
Peptidoglycan |
Carbohydrate |
Peptidoglycan is among the primary components of bacterial cell walls, having a supporting role in the form of mechanical properties of the wall. It is also involved in bacterial cell shape determination as well as in avoiding osmotic shock. |
TAQ 2
a) Involvement of enzymes and chemical reaction in human body
Catalase is a very important enzyme that detoxifies the cells through reducing hydroxide peroxide (H₂O₂), a compound found in the process of cell metabolism into water (H₂O) and oxygen (O₂). If not effectively neutralized, hydrogen peroxide becomes toxic that causes oxidative damage to the cells in the body. Catalase helps to catalyse this reaction so as not to harm the cells in an organism (Rial et al. 2021). Catalase is an enzyme that has a high catalysis rate to enable it to break excess hydrogen peroxide in cells promptly. This process is very important so that cells, especially liver and kidneys can effectively remove wastes from the body.
The chemical reaction is; 2H2O2 → 2H2O + O2
b) Function of enzymes in activation of energy
Although most enzymes act as specific catalysts by accelerating the rate of the chemical reactions, their most significant role is to reduce the activation energy needed for such reactions to occur. In other words, it is the amount of energy which can initiate a chemical reaction consisting of molecules of the reactant substances into products. This is through the interaction of the enzymes with special substrates at its active site, whereby it lowers the energy transition state. Enzymes thus facilitate the reactions to occur at physiological temperatures and conditions without getting used up in the process and thus can be used many times in the same cycle.
c) Induced fit Model
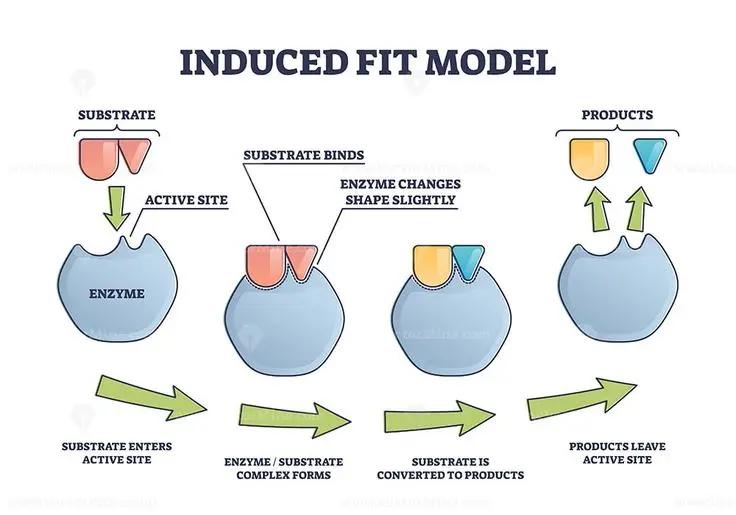
Figure 1: Induced Fit Model of Enzymes
The induced fit model describes how an enzyme’s active site also changes its shape in a large way to accommodate the substrate even more tightly. This concept is different from the earlier lock-and-key model which had put forward that the active part was a cavity having complementary shape to the substrate. It was the induced-fit model that said that an enzyme bends and changes its shape once the substrate binds to it (Lasker et al. 2022). This shows that when a substrate has approached and eventually binds to the active sector of the enzyme, the enzyme alters its shape slightly in order to improve the bond that is formed between the enzyme and the substrate. This conformal change allows the enzyme to encircle the substrate to form an enzyme-substrate that will reduce the stability of the transition state. In this way, the enzyme fixes this intermediate and makes the activation energy needed for the reaction to form lesser than that of its standard state. In other words, activation energy is the amount of energy required to initiate a chemical change, or to enable the breaking or formation of bonds. This lowers the energy barrier and therefore increases the rate of reaction thus making biochemical processes proceed at a fast rate when declaring enzymes (Zhao et al. 2021). After that, the enzyme can re-produce the neighboring molecule and protein and let go of the product or products that it was supposed to create. It can also be seen that to increase the enzyme in great specificity for the substrates, flexibility is required/there is a need for conformational changes. The induced fit model describes how enzymes uphold high specificity and also high efficiency in catalyzing biological reactions.
TAQ 3
Part 1
Law of Dominance
Mendel also discovered that the hybrids developed from the cross-breeding of pure breeding plants of two various characters, say, tall and dwarf plants, resulted into an F₁ generation in which all the progeny expressed the dominant trait, all being tall. This made it possible to deduce that those certain traits could be inherited dominantly or recessively.
|
P Generation (Parents) |
Offspring (F₁) |
|
TT (Tall) × tt (Short) |
Tt (All Tall) |
Table 1: Introduction of alleles
Law of Segregation
|
T |
t |
|
|
T |
TT (Tall) |
Tt (Tall) |
|
t |
Tt (Tall) |
tt (Short) |
Table 2: Punnett square
Mendel then crossed the F 1 hybrid plants of the type Tt with each other to get the second filial generation, F 2. He planted seeds from the tall plant and got three tall plants and one short plant; hence, the phenotypic ratio of 3:1 was established (Sagar et al. 2021). The shovel-shaped seedling marked by short-stemmedness appeared in ¼ (25 %) of F₂ plants which clearly indicated that both parents possess two alleles out of which one segregates during the formation of gametes.
|
RY |
Ry |
rY |
ry |
|
|
RY |
RRYY |
RRYy |
RrYY |
RrYy |
|
Ry |
RRYy |
RRyy |
RrYy |
Rryy |
|
rY |
RrYY |
RrYy |
rrYY |
rrYy |
|
ry |
RrYy |
Rryy |
rrYy |
rryy |
Table 3: Punnett square
Mendel also used two factors simultaneously, such as seed shape, which are Round (R) and Wrinkled (r) seeds, and seed colour, which are Yellow (Y) and Green (y). He crossed RRYY × rryy and noted that in the F₂ generation, the characteristics are segregated independently, which supports the 9:3:3:1 ratio.
Part 2 Sickle Cell Anemia
Sickle cell anaemia is a form of inheritance disease that results from the existence of a recessive gene, (s). Individuals with the SS genotype have normal haemoglobin, but persons with Ss are basically healthy; the ss genotype is associated with the disease (Saidolimovna, 2023). When both traits are SS, then he and she can pass the dominant allele S, or recessive allele s to the children. This gives a 25% of the child inheriting the SS gene, meaning ‘healthy’; 50% chance of the child inheriting the Ss gene meaning being a carrier but does not affecting the child; and only 25% of the child bearing ss gene causing Sickle Cell Anaemia (Schneider et al. 2021). This is in concordance with the topic of recessive genetic disorders in the context of Mendelian inheritance.
Carrier parents ‘conveys’ the disease when the mother and father are heterozygous for the gene, the probability of a child to have Sickle Cell Anaemia (ss) is 25% (1 in 4) and not to suffer 75% (3 in 4); this could be either SS or Ss. This is in line with Mendelian inheritance whereby recessive disorders are expressed only if the affected individual has inherited two recessive alleles.
Sickle Cell Anaemia (ss) is inherited, and if a sickle cell patient decides to bear a child with a healthy partner (SS), the child will inherit the S allele from the healthy partner and the s allele from the sickle cell patient. Hence, from the combination of ss × SS genotype, we derive a Punnett square showing that all the children will be negative Ss but will not be affected by the disease (Dai et al. 2023). As all children have a dominant S allele, the denominator is zero; thus the percentage of children who are sufferers (ss) is 0%. He was able to come up with the basic laws of inheritance dealing with the characteristics of genetics through experiments with pea plants. These principles intervene to explain genetic disorders such as Sickle Cell Anaemia in a way that shows how recessive alleles are handed down and how Punnett squares can be used in drawing probabilities.
TAQ 4
Part 1 Comparison of anaerobic and aerobic respiration
|
Points to Consider |
Aerobic Respiration |
Anaerobic Respiration |
|
Specific Pathways |
Electron Transport Chain, Glycolysis, Krebs Cycle |
Glycolysis, Fermentation |
|
Cellular Location |
Cytoplasm (Glycolysis), and Mitochondria (Krebs) |
Cytoplasm |
|
Oxygen Requirement |
Requires oxygen |
No requirement for oxygen |
|
Yield of Net ATP |
36-38 ATP/ glucose |
2 ATP/ glucose |
|
Additional Points |
Give co2 and H2o which is more efficient |
Give lactic acid in animals body or co2 and ethanol in yeast; less efficient |
Part 2
a) Significance of anaerobic respiration
They include anaerobic respiration which is essential in the generation of ATP for cells when oxygen is limited, ATP can only be produced through glycolysis. Here, NAD⁺ is regenerated in that pyruvate is converted to lactic acid to enable glycolysis to resume generation of ATP. Biomolecules such as NADH shall be regenerated so as to continue with ATP production. In fact, anaerobic respiration, though it yields only 2 ATPs per glucose molecule, is very vital in conditions such as in stepped up exercise, hypoxia or oxygen deprivation, lest total power failure ensue.
b) Cellular respiration
|
“Cellular Respiration Feature |
Glycolysis (Yes/No) |
Krebs Cycle (Yes/No) |
Electron Transport Chain (Yes/No) |
|
Involved in aerobic respiration |
No |
Yes |
Yes |
|
Occurs in the mitochondrial matrix |
No |
Yes |
Yes |
|
Pyruvate molecules are produced |
Yes |
No |
No |
|
Acetyl CoA combines with a 4-carbon molecule |
No |
Yes |
No |
|
Electrons are passed between protein carriers |
No |
No |
Yes |
|
ATP is produced |
Yes |
Yes |
Yes |
|
NAD⁺ gains hydrogen |
Yes |
Yes |
Yes |
|
FADH₂ loses hydrogen |
No |
Yes |
Yes” |
c) Biological function of oxygen and glucose
Glucose is the most important energy provider in the process of cellular respiration. It is derived from the dietary carbohydrates and is distributed in the bloodstream into the cells (Bohnsack et al. 2023). The metabolism of glucose commences with glycolysis taking place in the cytoplasm of cells that are found in the body. In this stage, glucose is split by the removal of two molecules of phosphate and the two resulting molecules of pyruvate make a net gain of two molecules of ATP and reduces NAD⁺ to NADH. After passing through glycolysis, pyruvate forms Acetyl-CoA and is transported to the mitochondria to experience another cycle known as the Krebs cycle where electrons with great energy are produced and carbon dioxide expelled as the waste product. The electrons produced through the oxidation of glucose are transferred by NADH and FADH₂ to the electron transport system. The last carrier or the final acceptor of electrons in the chain is oxygen. In the absence of oxygen, the whole aerobic process would come to a standstill since the electrons could not be offloaded from NADH and FADH₂ to continue the ATP production process. In the ETC, electrons are transferred to various protein structures located in the inner mitochondrial membrane. The movement of these electrons produces a proton gradient across the membrane and it facilitates the synthesis of ATP through oxidative phosphorylation.
At the terminal of the ETC, oxygen trapped the free electrons and combines them with protons to form water so as to avoid generation of free electrons that can harm cells. This is an important step to ensure that there is a constant generation of ATP within the cells. Aerobic respiration is quite efficient and the yield of ATP molecules is 36-38 per glucose molecule because this energy is needed for diverse cellular processes such as muscle contraction, active transport, and biosynthesis.
TAQ 5
Answer 1
|
“Biomolecule |
State the Biomolecule’s Class |
Provide a Description of the Biomolecule’s Structure |
Provide a Description of the Biomolecule’s Function |
|
Enzyme |
Protein |
Enzymes are globular proteins that are produced from a sequence of amino acids and are joined together by peptide bonds. They possess a definite tertiary structure containing a catalytic pocket into which the substrate can fit. |
These proteins are bio-catalysts that assist in speeding up chemical reactions within the body and are not used in the process. They reduce the activation energy for the metabolic reactions that take place in the body. |
|
Glucose |
Carbohydrate |
That is why the glucose is classified as monosaccharide or simple sugar that has six carbon atoms in its formula C₆H₁₂O₆. It comes in a linear form as well as in ring form and the later is more soluble in water. |
Glucose is the major energy provider in the cells of the human body. It is involved in glycolysis as well as cellular respiration in order to generate ATP that will power various cellular activities. |
|
Haemoglobin (Quaternary Structure) |
Protein |
Haemoglobin is a tetrapeptide formed by two alpha and two beta subunits which are polypeptide chains. The proline is in one chain and is coordinated with the haem group which is an atom with iron (Fe) attached to an oxygen atom. |
Haemoglobin is mainly responsible for the oxygen transport from lungs to tissues and tissue transcription of carbon dioxide to lungs for exhalation (Chowdhary, and Tank, 2023). |
|
Phospholipid |
Lipid |
Phospholipids are composed of glycerol molecules, two hydrocarbon tails, and a phosphate with a charge, making it also hydrophilic. This property makes them fold into bilayers easily. |
They are the key constituents of the biological membranes being the primary component of the lipid bilayer that controls the movement of materials across membranes.” |
Answer 2
a) Structure A is the Amino acid. Amino acids are among the natural organic compounds that form the proteins. They are characterized structurally by having an amino (-NH2) group, a carboxyl (-COOH) group, and a hydrogen atom and a side-chain group (R) covalently bonded to a central carbon atom (Conti, and Oppikofer, 2022).
b) Here the bond X is the peptide bond. A peptide bond is a covalent link between the carboxyl groups of one amino acid with the amino group of another under a condensation reaction, which gives out water.
c) i) A protein structure that can be described on the molecular level is the primary structure, which is the amino acid sequence of a protein linked by peptide bonds in a linear fashion. This sequence is responsible for the folding of the protein and all its activities. It may be described as a point mutation since any alteration in the amino acid sequence can have drastic consequences on the protein’s biological functionality; a clear example is sickle cell anemia.
ii) Tertiary structure can be described as the folding of a protein in the third dimension caused by the contacts between R-groups of particular amino acids. These include hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions, which are very vital in the stability and functioning of the protein (Mitrea et al. 2022). A named example is catalase, which is an enzyme existing in cells, and its function is to decompose hydrogen peroxide (H₂O₂) that exists inside cells into water and oxygen in order to prevent the destruction of cells due to oxidation. Another is haemoglobin, a protein that occurs as a well-defined tertiary structure able to uptake and transport oxygen in red blood cells.
Answer 3
a) Molecule B is glucose and this is a monosaccharide or simply known as a basic sugar which the cells of the organism use for energy. Its chemical formula is C₆H₁₂O₆ and it has two different forms: alpha (α) and beta (β) glucose in terms of conformation of the hydroxymethyl group at the first carbon atom.
b) A disaccharide made up of glucose is referred to as maltose and its molecular formula is C₁₂H₂₂O₁₁. It is a polymer of glucose in which two molecules of glucose are joined together through a covalent α-1, 4-glycosidic bond formed by the elimination of a water molecule by a dehydration synthesis process (Kirschbaum, and Zwicker, 2021). For energy production, maltose is converted by the maltase enzyme into glucose. Another example of an O glycoside is sucrose which is made of glucose and fructose attached together by α 1, 2 glycosidic bonds. Sucrose is the sugar which is used daily and also a vascular or transported sugar in plants.
c) In the RNA nucleotides, the pentose sugar used is ribose. It is an organic molecule with the chemical formula C₅H₁₀O₅, and it has the role of connecting phosphate groups and nitrogenous bases to establish nucleotides such as adenosine triphosphate (ATP) and ribonucleic acid (RNA).
Answer 4
i) Cholesterol is a sterol lipid that contains four rings in its ring structure system; three are hexagonal rings and the other is a pentagonal one, generally referred to as sterol nucleus. It has a polar functional group, the hydroxyl (-OH) group which has the ability to make it both hydrophilic at one end and a fatty hydrocarbon at the other end that makes it able to interact with the phosphate bilayers within the membranes (Schuster et al. 2021). Cholesterol being synthesized in the liver is an essential lipid component that is used in the synthesis of steroid hormones such as testosterone, estrogen, bile acids, and vitamin D.
ii) Cholesterol has various functions one of which is to help in regulating the fluidity of the cell membrane. At high temperatures, it decreases fluidity and thus discontinues uncontrolled and uncontrolled movement of membranes. In low temperature, the terms ward off such effects as membrane stiffening to allow flexibility. Also, it plays a role in forming lipid rafts that are vital in cell signaling and organizing proteins in cells that are in the membrane. Therefore, cholesterol is essential for cell membrane, signal transduction and hormone biosynthesis.
Reference materials and sample papers are provided to clarify assignment structure and key learning outcomes. Through our Assignment Help UK, guidance is delivered while maintaining originality and ethical academic practice. The Biomolecules and Cellular Functions Assignment Sample demonstrates practical understanding of proteins, carbohydrates, lipids, enzymes, and cellular processes.
References
- Alberti, S. and Hyman, A.A., 2021. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nature reviews Molecular cell biology, 22(3), pp.196-213.
- Alderson, T.R. and Kay, L.E., 2021. NMR spectroscopy captures the essential role of dynamics in regulating biomolecular function. Cell, 184(3), pp.577-595.
- Bohnsack, K.E., Yi, S., Venus, S., Jankowsky, E. and Bohnsack, M.T., 2023. Cellular functions of eukaryotic RNA helicases and their links to human diseases. Nature reviews Molecular cell biology, 24(10), pp.749-769.
- Chowdhary, V.A. and Tank, J.G., 2023. Biomolecules regulating defense mechanism in plants. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 93(1), pp.17-25.
- Conti, B.A. and Oppikofer, M., 2022. Biomolecular condensates: new opportunities for drug discovery and RNA therapeutics. Trends in Pharmacological Sciences, 43(10), pp.820-837.
- Cook, A.B., Novosedlik, S. and van Hest, J.C., 2023. Complex coacervate materials as artificial cells. Accounts of Materials Research, 4(3), pp.287-298.
- Dai, Y., Farag, M., Lee, D., Zeng, X., Kim, K., Son, H.I., Guo, X., Su, J., Peterson, N., Mohammed, J. and Ney, M., 2023. Programmable synthetic biomolecular condensates for cellular control. Nature Chemical Biology, 19(4), pp.518-528.
- Kirschbaum, J. and Zwicker, D., 2021. Controlling biomolecular condensates via chemical reactions. Journal of The Royal Society Interface, 18(179), p.20210255.
- Lasker, K., Boeynaems, S., Lam, V., Scholl, D., Stainton, E., Briner, A., Jacquemyn, M., Daelemans, D., Deniz, A., Villa, E. and Holehouse, A.S., 2022. The material properties of a bacterial-derived biomolecular condensate tune biological function in natural and synthetic systems. Nature communications, 13(1), p.5643.
- Lyon, A.S., Peeples, W.B. and Rosen, M.K., 2021. A framework for understanding the functions of biomolecular condensates across scales. Nature reviews Molecular cell biology, 22(3), pp.215-235.
- Mitrea, D.M., Mittasch, M., Gomes, B.F., Klein, I.A. and Murcko, M.A., 2022. Modulating biomolecular condensates: a novel approach to drug discovery. Nature Reviews Drug Discovery, 21(11), pp.841-862.
- Rial-Hermida, M.I., Rey-Rico, A., Blanco-Fernandez, B., Carballo-Pedrares, N., Byrne, E.M. and Mano, J.F., 2021. Recent progress on polysaccharide-based hydrogels for controlled delivery of therapeutic biomolecules. ACS Biomaterials Science & Engineering, 7(9), pp.4102-4127.
- Sagar, N.A., Tarafdar, S., Agarwal, S., Tarafdar, A. and Sharma, S., 2021. Polyamines: functions, metabolism, and role in human disease management. Medical Sciences, 9(2), p.44.
- Saidolimovna, K.S., 2023. STRUCTURE OF THE ENZYME AMYLASE, FUNCTION IN THE BODY, MECHANISM OF RECIRCULATION. Journal of Social Sciences and Humanities Research Fundamentals, 3(05), pp.40-42.
- Saxena, P., Selvaraj, K., Khare, S.K. and Chaudhary, N., 2022. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnology letters, pp.1-22.
- Schneider, A.F., Kithil, M., Cardoso, M.C., Lehmann, M. and Hackenberger, C.P., 2021. Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives. Nature chemistry, 13(6), pp.530-539.
- Schuster, B.S., Regy, R.M., Dolan, E.M., Kanchi Ranganath, A., Jovic, N., Khare, S.D., Shi, Z. and Mittal, J., 2021. Biomolecular condensates: Sequence determinants of phase separation, microstructural organization, enzymatic activity, and material properties. The journal of physical chemistry B, 125(14), pp.3441-3451.
- Zhao, M., Ma, J., Li, M., Zhang, Y., Jiang, B., Zhao, X., Huai, C., Shen, L., Zhang, N., He, L. and Qin, S., 2021. Cytochrome P450 enzymes and drug metabolism in humans. International journal of molecular sciences, 22(23), p.12808