Reactor Design For Propane Dehydrogenation
Design and kinetic analysis of a plug flow reactor for propane dehydrogenation to propene under Project CEE_6_DEP, using advanced modeling, control, and simulation techniques.
Ph.D. Experts For Best Assistance
Plagiarism Free Content
AI Free Content
Introduction
The dehydrogenation of propane to propene is a major industrial process in the chemical industries since it is an intermediate process in the generation of many chemicals such as plastics, fibers, amongst others in the petrochemical industries. Propene is mainly an intermediate to a number of chemicals and playing major role in the production of polypropylene, which is a broadly used plastic. The present report aims at designing a Plug Flow Reactor (PFR) for the directly conversion of propane to propene; a process that is endothermic in nature and therefore requires significant heat and mass integration. The reaction has been found to be second order with respect to propane and obeys the first order kinetics with respect to oxygen. In the operating conditions of 650°C and 1 bar pressure, the conversion of propane should be as high 95%, and the selectivity for the formation of propene should be 100%. The reactor design provides the maximum heat and mass integration so as to achieve high efficiency, safety, and utilization of energy with the desired product formation. From the Brownrigg diagram, it can be seen that one area that has a notorious impact on the practicality of the reactor as well as the conversion efficiency of the propane is the reactor volume and the residence time it offers. The reactor’s volume is selected to be 100 m³, while the residence time is estimated to be 420 seconds, which is almost 30 minutes to ensure that the propane gets enough time to interact with the catalyst. This long residence time guarantees that the reaction attains the intended conversion though using less energy. Furthermore, the elements of the reactor system also involve temperature control because the reaction is temperature-sensitive. For purposes of saving energy the temperature had been reduced from 650°C to 600°C in the axial direction of the reactor. For assessment of the performance of the reactor, recording of propane concentration throughout the reactor’s length, temperature distribution, reaction rates and conversion is carried out using MATLAB simulations. The demand of propene has been frequent in the past several years at an estimated growth rate of 3.6% in the global market. This growth is however supported mainly by the increase in the polypropylene market that covers up to 65% of the entire propene demand. The traditional ways of propene synthesis are steam cracking of naphtha and fluidized catalytic cracking (FCC) of heavy oils. Yet, the four methods have some shortcomings in selectivity and ecological releases. Direct dehydrogenation of propane has a potential of higher selectivity and lower carbon impact hence can be considered attractive.
Reactor Design: Reaction Kinetics
The propane dehydrogenation reaction (C₃H₈ → C₃H₆ + H₂) represents the conversion of hydrogen propane to propene. This endothermic reaction is carried out by using PtSn/Al₂O₃ catalyst and the reaction is found to be first order with respect to propane. The rate of a reaction at which it proceeds is given as rₐ where rₐ = k·Cₐ for propane, with k as the rate constant, and Cₐ as propane concentration measured in mol/L. The first order dependency (n=1) means here that the rate of the reaction is causing a direct proportionality in the concentration of propane. When at the operating conditions of 650°C pressure of 1 bar the rate constant k is calculated from the Arrhenius equation which depicts the dependence on the temperature (Gambo et al., 2021). This kinetic model helps in determining other values proximately to the design of the PFR which include reactor volume, residence time and conversion profile. This is important because it helps in controlling the performance of the reactor in order to obtain the desire 95% conversion coupled with 100% selectivity to propene. The reaction kinetics of propane dehydrogenation (C₃H₈ → C₃H₆ + H₂) has first-order with regards to the propane concentration (Cₐ), so the rate, rₐ = k·Cₐ and the rate constant (k) depends on the temperature that is given by the Arrhenius equation.
The Arrhenius equation may, therefore, be written as A = Ae ^ (-Ea/RT)
k=A⋅e^−Ea/(R⋅T)
Where:
k is the rate constant (s⁻¹), An is the pre-exponential factor and is expressed in reciprocal of seconds, E₍a₎ is the activation energy and is normally expressed in Joule per mol, R is the specific gas constant which is common for all gases (8.314 J/mol·K), T here T is the absolute temperature in Kelvin which is the measure of the statistical temperature.
In the case of PtSn/Al₂O₃ for a dehydrogenation reaction, the activation energy, E₍a₎ is found to be equal to 136 kJ/mol, and the pre-exponential factor, A is equal 3.5 × 10⁹ s⁻¹. Thus, the rate constant at the operating temperature of 650°C (923K) can be given as:
K = 3.5×109⋅e^−136,000/(8.314⋅923) = 0.0524 s−1
This rate constant value is very important in the assessment of the reactor performance and sizing.

Figure 1: Langmuir-Hinshelwood mechanism
The proposed mechanism that has been put forward in the course of the study of propane dehydrogenation is the well-known Langmuir-Hinshelwood mechanism and it has the following sequence of steps:
Adsorption of propane on catalyst surface:
C₃H₈ + S ⇌ C₃H₈-S
Surface reaction:
C₃H₈-S → C₃H₆-S + H₂-S
Desorption of propene:
C₃H₆-S → C₃H₆ + S
Desorption of hydrogen:
H₂-S → H₂ + S
Where S is an open active site on the surface of the catalyst.
This suggests that a more extensive rate equation can be deduced from the model based on the Langmuir-Hinshelwood mechanism:
ra= (k1KC3H8PC3H8−k1′KC3H6KH2PC3H6PH2)/(1+KC3H8PC3H8+KC3H6PC3H6+KH2PH2)2
Where:
ra is the reaction rate
These constants will be referred as surface reaction rate constant and will be noted ks
K{C3H8}, K{C3H6}, and K{H2} are adsorption equilibrium constants
P{C3H8}, P{C3H6}, and P{H2} are partial pressures
The reverse reaction rate constant is therefore denoted as k1’.
According to the given operating conditions of our reactor design, temperature 650°C, pressure 1 bar, the value of the parameters are as follows:
K1 = 5.24 \times 10^{-2} mol/(g·cat·h)
K{C3H8} = 0.65 bar⁻¹
K{C3H6} = 0.31 bar⁻¹
K{H2} = 0.08 bar⁻¹
These kinetic parameters were obtained. From the conversion data, using regression analysis at different operating conditions.
The equation was established with the help of the Langmuir-Hinshelwood mechanism that contains steps of adsorption, reaction and desorption occurring on the surface of the solid catalyst. This equation is a complex form of rₐ = k·Cₐ. The rate expression is expressed from the adsorption isotherm which define the surface coverage of propane, propene, and hydrogen with the help of constants. When these coverages are introduced to the reaction rate equation, a comprehensive rate law is established with competitiveness of adsorption and the driving forces of the reaction. This equation then takes into consideration of the catalyst surface, inhibition and the orders of reaction making a mathematical model than simple kinetic model.
Advanced Kinetic Studies
In particular, the use of extended kinetic studies as a research approach yields the precise data on the mechanism of propane dehydrogenation process. These are the methods such as carry through spectroscopy and isotopic labelling used to identify the course of the reaction and of theintermediates (Matthies et al., 2024). For this reason, understanding the reaction mechanism in detail will help the researchers introduce the improvements with the catalyst and the reactor to enhance the conversion and selectivity of the process.
Catalyst Surface Interactions
As it can be found out from the above explanation the degree of interaction of the catalyst with the reactant species determines the rate of reaction. There are various analysis techniques like those of STM and AFM that are helpful in giving information concerning the adsorption and desorption process that happens on the surface of the catalysts (Kumar and Srivastava, 2023). These aspects make it easier to modify the surface properties of the catalyst to enhance his/her efficiency in the dehydrogenation process.
Influence of Temperature and Pressure
One of the important factors of the problem is the conditions inside the reactor, which may include temperature and pressure. Higher temperatures are beneficial to the endothermic dehydrogenation reaction of propane since the energy is required for the breaking of the C-H bonds is supplied. But if the temperature is too high, there will be unwanted side reactions that include cracking or production of coke which may deactivate the catalyst. Consequently, it is very important that it is kept within a certain range of ideal temperature (Zhao et al., 2021). Consequently, pressure affects either the adsorption or desorption of various elements within the catalyst as well. Less pressure can help in desorption of products in order to minimize the possibilities of secondary reactions in the system.
Catalyst Deactivation and Regeneration
Another factor that makes catalyst deactivation an issue in propane dehydrogenation is the buildup of coke deposits on the specific surface of the used catalyst. Coke formation covers the active sites hence the overall efficiency of the catalyst would be greatly affected over a long time. To avoid this, there are regeneration processes carried out on the catalyst whereby the coke builds is burnt off so as to regain its activity again. This knowledge is of high importance if the kinetics of coke formation as well as the conditions that enable coke formation are to be understood and effectively addressed.
Experimental Techniques for Kinetic Studies
Temperature-Programmed Desorption (TPD)
Temperature-Programmed Desorption (TPD) is one of the most commonly used experimental techniques for the studies of adsorption and desorption on the catalyst surface. This is done by heating the catalyst in a controlled condition and determining the amount of gas desorbed at a given temperature. The gathered information assists in elucidating characteristics of adsorbed species as well as bonding strength with the catalyst and participation of species in a reaction mechanism.
Temperature-Programmed Reaction (TPR)
Temperature-Programmed Reaction (TPR) is a method employed to study kinetics and process of propane dehydrogenation reaction. Here the catalyst is taken through the following steps; the reactant gas is introduced and the reaction is done with gradual rise of temperature. This is because the reaction rate at different temperatures gives insight on the activation energy, the reaction intermediates as well as the stability of the catalyst when subjected to the reaction conditions (Yu et al., 2023). This is through the gained insight on the effects of temperature on the catalytic process as provided by TPR.
Isotopic Labeling
Isotopic labeling is an effective method where one or more of the reactants are prepared with isotopes of one or more elements in the molecule that is being labeled so that the pathway and the intermediate products formed in a chemical reaction can be easily identified. Due to labeling, atoms of the reactants can be replaced with isotopes namely carbon-13 or deuterium enabling study of the mobility of atoms in the reaction process (Brune, 2024). This technique helps to determine the reaction mechanism, reactivity of intermediaries and establishment of the involvement of certain sites of the catalysts.
In Situ Spectroscopy
In situ spectroscopy, with methods such as Infrared (IR) and Raman spectroscopy, is used characterize the catalyst surface under working conditions. The periodic table chalcogen-based compounds complemented with calculations of Density Functional Theory showed that the several adsorption sites, reaction types and chemical bonds’ nature can be seen with the help of IR and Raman spectroscopy, concerning a wide range of investigations of the catalyst material.
Degree of freedom
The analysis of the degree of freedom (DOF) of the propane dehydrogenation process for a Plug Flow Reactor (PFR) involves looking at the number of independent variables and the number of equations that are present in the system. Some of the most commonly used independency variable are factors such as temperature, pressure flowing rate and concentrations. The constraints can be assumed from mass balance equations, energy balance and rate equations. Finally it gives the degrees of freedom which is the number of independent variables that one can change after the constrained have been taken into consideration . As a matter of fact, in a classical PFR, approximation shows that DOF is equal to 1, so just one variable has to be regulated or controlled.
Reactor Volume and Residence Time
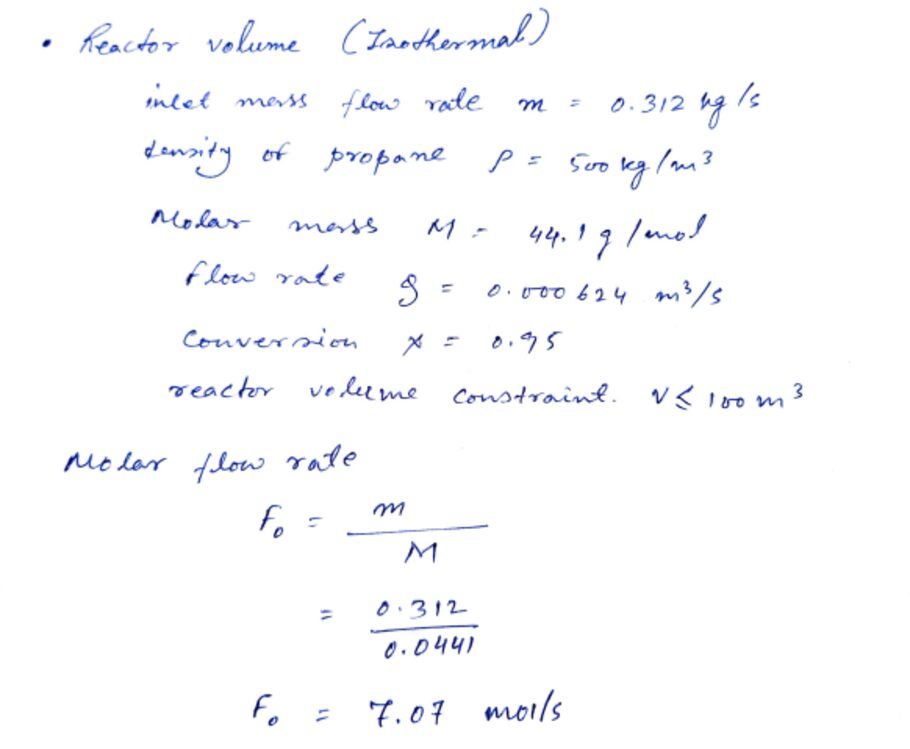
Figure 2: Reactor Volume
The other important element in PFR design is the space velocity or the residence time (τ) which describes the time the reagents take in the reactor. This parameter directly influences conversion and is expressed as τ = V/Q, the volume of the reactor, given in liters, and the volumetric velocity Q in liters per second. For the reactor for propane dehydrogenation, the volume has been selected as 100 m³because reaction kinetics and specific conversion rates are taken into consideration. With the volumetric flow rate of propane feed being calculated, it is possible to maintain the residence time of the propane at 420s which is equivalent to thirty-seven minutes and twenty two seconds. This prolongation of their stay in the reactor allows for a long contact time to ensure that the propane interacts with the PtSn/Al₂O₃ catalyst to the desired 95% conversion. This required reactor volume was achieved taking into account other factors which include pressure drop, heat transfer area and catalyst inventory (Oyegoke et al., 2024). Due to the tremendous importance of these parameters, their optimization is very crucial to give enhanced propane conversion when the process is economically feasible at the same time. It also highlights essential factors for the operation of a Plug Flow Reactor (PFR) used in propane dehydrogenation, such as the size of the reactor, 100 m³, the flow rate (26942.89 kg/day), the residence time of 420s and the conversion efficiency of 95%.
The reactor volume for a non-isothermal reactor could therefore be determined from the reaction kinetics coupled with the energy balance equations. It depends with the heat that is produced or absorbed by the reaction which dictates the temperature gradient in the reactor. Based on the energy balance equation, the heat of reaction, heating and cooling losses and the heat transfer either into or out of the reactor is calculated. The need to take into account the variation of temperature leads to the need to determine the temperature distribution whilst setting the area of the reactor in accordance with the desired conversion and mean residence time at different temperatures.
Residence time should be kept as optimal as possible in order to achieve high selectivity towards the desired product propylene and reduce the rate of side reactions such as cracking or coke formation. This would ensure that the reaction is carried out at the desired temperature and pressure so as to avoid degradation of the catalyst and to achieve the desired percentage conversion. Additionally, information on suitable catalysts for the implemented reaction and their topographical distribution also influence the usefulness of the reactors. By incorporating flow dynamics and heat transfer at its optimum levels, the reactor will perform optimally without much threat to process economy. Overall, high space velocity, residence time and catalyst utilization result to high production for the reactor and efficient industrial use.
Optimization of Reactor Geometry
Geometry of the reactor is an important factor to be considered when designing the propane dehydrogenation reactor. These characteristics have a great impact on the duration of presence of the reactants inside the reactor and the manner in which they flow through the system. A longer reactor gives extra time to conversion of reactant with catalyst and thus higher conversion rates may be achieved. However, when the length of the reactor is too long, there will be pressure drop and non uniform flow which is likely to affect the efficiency of the reaction. Computational Fluid Dynamics (CFD) is one of efficient tools used to optimise the field of flow existing inside the reactor. Such principles of study under the different geometries help in deciphering the problem zones of pressure drop and flow uneven distribution by the use of CFD. This prevents channels appears which in turn helps in maintaining the flow filed throughout the reactor and makes it easier for the reaction to occur under the required conditions (Motagamwala et al., 2021). Various parameters involved in the reaction could be controlled by the geometrical characteristics of the reactor with the aim of increasing the rate residence time to increase the efficiency of dehydrogenation process.
Dynamic Flow Control
Dynamic flow control systems are crucial to the overall management of reactant residence time and ensuring that the distribution of the reactant is adequate within the reactor. These systems use feedback control software to control the flow rates and also to regulate them depending on operating conditions. Through flow control dynamics, the reactor can be made to run at the simplest reaction rate, in terms of conversion and selectivity and with least energy input. An accurate control of residence time and distribution of reactants is a key characteristic of dynamic flow control systems in a reactor. These systems apply high feedback control software to maintain the flow rate and adjust it based on the new changes in the operating conditions. Dynamic flow control systems can help sustain the appropriate reaction conditions by keeping the flow rates as variable, this would warrant high conversion and selectivity with minimal energy consumption. Dynamic flow control is most relevant in corner cases like propane dehydrogenation mainly because of controlling the flow rates of the reactant. These systems can sense temperature, pressure and concentration of the reactants and can regulate the flow rate accordingly at any particular time (Ingale et al., 2021). This also increases the effectiveness of the reaction and also plays the part in the stability of the process as a whole.
Heat Transfer Considerations
Heat transfer becomes an influential parameter that control and maintain the temperature gradient across the reactor; this is especially significant in enhancing reaction rates in non-isothermal systems. Heating is done in such a manner as to ensure that the temperature at which the reaction occurs is at its most favorable because this will increase the conversion level and selectivity. There is also heat transfer coefficients which refer to the manner in which the heat is transferred in and out of the reacting mixture and the design of the reactor has to consider this factor. The position of heaters or coolers has to be properly done so that there is even distribution of heat or coolness in the reactor. Placement of heating coils inside or outside jackets helps in controlling the temperature and hot or cold spots that are undesirable for the reaction to occur. Moreover, the ability of transferring heat through the reactor structural and inner materials contributes to thermal conductivity. The heat transfer media and other materials employed in manufacturing materials with high thermal conductivity make it easy to regulate the temperature all across the reactor (Li et al., 2023). This is specifically true in large-scale reactors wherein the temperature differentials cause uneven uptake of reactions and therefore low effectiveness.
Catalyst Utilization and Distribution
One of the most important factors that influence the residence time and the conversion characteristics is the manner the catalyst is dispersed within the reactor. Uniform distribution and amount of the catalyst are important due to the actual contact that the reactant species have with the catalyst to facilitate the improvement of the rate of reaction. Some of the procedures under this category include bed profiling, which aids in the uniform distribution of the catalyst within the reactor. It helps to avoid concentrations of heat which may eventually deactivate the catalyst and also ensures equal reaction rates. The employment of well-defined catalyst supports including monolithic or honeycomb supports can be still more advantageous for catalyst use because of the increased area of reaction and effect of better convective diffusion (Ghasemigoudarzi et al., 2021). These features and characteristics mean that the structure is effective in aspect of flow distribution and pressure drop leading to improved performance.
Mass and Energy Balances
The weight of the catalyst is determined through the volume, density, and the loading fraction in the reactor. The values of reactor volume were 0.262 m³, the density of the catalyst was 1.2 g/cm³, the catalyst loading fraction was 10%, then calculating the weight of the catalyst (Wc) it was identified as 31.44 kg. Suppose this calculation to make sure that the reactor is filled with just the right amount of catalyst that is required for operation. mass of the catalyst is proportional to the reactivity of the reactor in a set with regard to such parameters as reaction rate the heat transfer.
The conservation of mass for propane and propene implies that it is the amount of mass that enters the reactor will be equal to the amount of mass which exits the reactor. Given the flow rates: Inlet propane flow rate: 26,942.89 kg/day, Outlet propane mass flow rate: 1347.14/100 = 13,3 kg/day (unreacted propane). Propene produced: 580 mol/day.
So, as converted is 95%, still, 30.5 mol /day of propane is unconverted. The rest of propane gets converted into propane according to the 100% selectivity contemplated for this reaction path. In order to calculate temperature distribution in the reactor, the energy balance is reduced as follows. Although a more refined approach could include the estimates of specific reaction enthalpies and heat losses, for the purpose of simplification the linear temperature profile from 650°C to 600°C for the reactor length was assumed (Carter et al., 2021). This makes sure that the reactor works at the temperatures that are desired so as to maximize the tendency of the reaction rates.
Detailed Energy Balance Analysis
Energy balance assessment is considered to be important to quantify the heat transfer phenomena occurred in the reactor. The propane dehydrogenation reaction is an endothermic reaction – this is why it demands a large amount of heat to take place. This entails getting the correct rate of heat input which is necessary in pertaining specific degree of temperature and the rate of conversion of the reaction. This way the engineer manages to ascertain where heat losses and other inefficiencies within the energy flow are likely to occur. This energy balance differs from the heat of heat of reaction, heat losses through the reactor wall, and the heat required to raise the reactant temperature to the reaction temperature. This task is usually achieved using complex simulation tools and computational models that offer a better understanding of the heat distribution and use in a reactor (Wang et al., 2022). By making Heat balance, the engineer can design the reactor in such a way that; there will be a minimal loss in the process and most of the energy that has been used to advance the reaction will be enough.
Integration of Heat Recovery Systems
Implementing heat recovery within the designs of the reactors can improve the efficiency of heat integration to a great extent. These are meant to recover and utilized in the regeneration processes, the heat produced during dehydrogenation reaction to minimize energy use. Heat recovery systems generally apply heat exchangers to transfer heat from the hot reaction products to the reactants, thus warming the latter up prior to their introduction to the reactor. Some of the advantages of adding the heat recovery systems includes that the reactor will be able to operate more sustainably. Such heat can also be utilized within other processes of the plant to increase the general efficiency of the plant activity (Huš et al., 2021). Thirdly, there is the aspect of cost since energy is among the critical operational needs; loss reduction means less money spent on its procurement.
Detailed Energy Balance Calculations
Heat of Reaction
The first step which is used in an energy balance calculation is the heat of the reaction, which is the heat that is needed for the dehydrogenation process. This is done with the help of enthalpy change (ΔH) of the reaction taking place in a given solution. The standard enthalpy of formation for both the reactants and products is calculated and then heat of reaction is obtained with the help of enthalpy change paragraph between the given products and reactants (Li et al., 2024). The heat of reaction gives a measure of amount of heat evolved or absorbed in the reaction thus posing a significant aspect in regulation of temperature within the reactor.
Heat Losses
Then, the heat losses from the reactor are determined. These losses normally take place through the reactor case and any other surrounding surface of the unit. The amount of heat loss can be calculated from heat transfer coefficients as well as the difference in temperature from the reactor to the surroundings. Some reasons for the heat loss include insulation properties of the reactor, ambient temperature, and designing of the reactor.
Heat Input
Therefore, after subtracting these losses, then the heat to be supplied to the reactor that will sustain the general temperature is determined. This would comprise the heat for the dehydrogenation of the alkylbenzene, and the heat needed for heat losses (Zhao et al., 2022). The heat input is usually from a heater or furnace used in the process, and the capacity of the heater or the heating system has to be determined on the basis of the total heat requirement.
Temperature Profile
Lastly, temperature profile in the reactor length direction is defined. Optimization here entails determination of the temperature of the energy balance equation at the different time intervals of the reactor operation for a controlled temperature regime. From the flow rate it is therefore possible to optimize the heat which is being supplied in order to maintain the ideal reaction conditions.
Advanced Control Systems for Energy Management
Stable and efficient control of the energy balance is an important task and for this purpose advanced control mechanisms are required in the reactor. It involves the use of sensors and sophisticated control algorithms that allows for tracking and controlling temperature, pressure, and flow rate of the reactor. These parameters are therefore controlled all the time to ensure the reactor operates in the best conditions possible, high conversion and selectivity, low energy use. Altogether, mass and energy balances are crucial to the synthesis and implementation of a propane dehydrogenation reactor (Zhang et al., 2023). Thus, by optimizing these factors engineers can enhance the performance of the reactor with lowest energy consumption and demonstrate great longevity.
Piping and Instrumentation Diagram (P&ID)
The P&ID of the reactor shows the feed and product lines together with control valves, temperature indicators and pressure control units. This block should help to comprehend the flow through the process and the control to sustain the desired conditions in the reactor (Martino et al., 2021). This ensures the temperature, the pressure as well as the flow rates are well regulated in order to have the reactions in check. In the diagram, the control system of an R-100 reactor is disclosed. Cooling inlet and outlet, flow control valves CV-1, CV-2, CV-3, level transmitter LT-01, LT-02 and temperature transmitter TT-01, TT-02 are some parts of the system. The flow rate control and pressure control are both controlled and regulated and the liquid is heated, cooled and mixed before exiting the reactor at the right temperature, and to ensure safety the system has a shutdown valve (SDV-2).
Advanced Control Systems
There is always a need for some form of sophisticated control mechanisms in order to ensure the continuance of efficient and effective operating conditions within the reactor. These systems employ various sensors and control bolometer to detect and control temperature, pressure and flow rates continuously (Zhang et al., 2022). These conditions are important in influencing the flow rate of the reactants because the process of dehydrogenation is endothermic and requires an accurate amount of heat. Sophisticated closed-loop control can be accomplished to alter germane heating components as heat exchangers to attain precise thermal profile for sustainable reaction conditions. Pressure also affects the reaction rate and the selectivity of the reaction, thus the pressure control is equally critical. Modern electric control systems include pressure transmitter and control valves for pressure control of the reactor and these react quickly to any change (Hannagan et al., 2021). Flow control is done using flow meters and control valves which regulates the flow rate of reactant to the reactor to achieve the required conversion and selectivity. These advanced control systems allow the reactor to run under conditions that provide maximum conversion and selectivity with low energy usage and tendency to upset the process.
Safety and Emergency Shutdown Systems
Some of the safety and emergency shutdown systems are very crucial as they are in-built to prevent the occurrence of safety issues during the dehydrogenation process of the reactor (Walter et al., 2021). Some of these systems consist of pressure relief valves such that whenever the pressure builds up beyond the required limit the extra pressure is safely released out of the system, the second one is emergency shutdown valves that can stop the flow of reactants at any time. Safety interlocks are also incorporated into the system so as to halt the process operation where it is unsafe for the process units through closure. Through high safety and shutdown systems, the reactor will be safe thus reduce on-the-job accidents to protect human beings and machinery (Zhang et al., 2023). These systems are essential for the functionality of the plant and essential for meeting its safety requirements for the dehydrogenation process.
Importance of P&ID in Reactor Design
A P&ID is an important tool in the design as well as in the management of a chemical reactor since it depicts the exact details of the piping, instrumentation, and controls to be used. That means it is needed to design, build, operate or manage this type of reactor system for the needed purpose. The P&ID is used to facilitate the verification of installation of other components of the system as well as their correct functioning in regard to safety and efficiency.
Key Components of a P&ID
Piping Layout: The P&ID also shows all pipes, their dimensions, materials required and the directions of the flow. Thus, it becomes evident that the reactants’ concentration and products is maintained in the required range within the system. The piping layout also includes valves, which are to direct the flow of materials and act as a barrier to part of the system at a time for purposes of checking or repairing and the like.
Control: The P&ID also defines the place and kind of instruments that is needed to control the process. Some of the mounted instruments consist of; temperature sensors, pressure gauges, flow meters and level transmitters (Yonge et al., 2023). These are devices that offer crucial information that is required in order to have the best working conditions and more to do with safety.
Control Systems: This shows the control systems which control various process parameters including temperature, pressure and flow among others. Control valves, controllers, and actuators are incorporated in the system such that it will modify these variables through feedback from the instruments.
P&ID Specifications: Some of the P&ID specification includes relief valves, shut down valves and emergency shut down systems. These features are laid in such a way that they act as barriers to accidents and also as means to reduce the consequences of the failures or the malfunctions. Role of P&ID in Process Optimization
Due to the fact that it interconnects the two-phase flow of the reactants, the P&ID is very important in determining the efficiency of the reactor system. As such, the P&ID enables the engineers to see the preventive bog, weak points, and opportunities for the development within the process layout clearly and in details (Zhang et al., 2024). This information will help to enhance the design and operation a reactor in such a way that will make it operate more efficiently at reduced cost and with improved safety.
Integration with Automation Systems
In contemporary reactor systems, P&ID may be linked with the advanced automation systems like Distributed Control Systems (DCS) or Supervisory Control and Data Acquisition (SCADA) systems. These systems work with the help of information provided in the P&ID to get the real-time data about the process to ensure the proper functioning of the reactor. The automation systems also have built in data logging and analysis, which is useful in diagnostics, maintenance and optimization.
Maintenance and Troubleshooting
A P&ID is one of the best documents a maintenance department and troubleshooting can be endowed with. It summarizes the system configuration and helps maintenance officers and electricians to easily identify specific elements that require some attention. The P&ID also plays a major role in the identification of problems since it visually illustrates the flow of the material and signal in the system (Qi et al., 2021). This information is important from the point of calling in order to fix a problem and reduce time wasted on that problem.
Regulatory Compliance
The specification is usually needed for the end user’s compliance purposes because the P&ID provides proof that the reactor system is safe and enironmentally friendly. Some of the areas that are checked in a P&ID include; Regulatory bodies are usually used to ascertain that the system is as per the laid down laws or not (Yang et al., 2025). This also helps in providing personnel and environmental safety since the reactor system is protected from both sides of the covers.
MATLAB Analysis and Plots
The Simulink model concerns with a reactor system which has the manipulatives of temperature and output flow rate for the system. It starts with the Flow Rate input which determines feeding rate of material into reservoir or any other equipment of the reactor. This flow is controlled by the help of the Flow Control Valve; this helps in controlling the input flow according to the control signal. The Reactor Dynamics block calculates the reactor’s thermal output based on a first-order transfer function that can simulate the heat changes over a reactor when the inputs change. Once the temperature is measured, a comparison is made with the set temperature and an Error Signal is produced which shows how much from the set temperature actually is the temperature. For better control, any high frequency noise in the temperature signal is removed by a Lowpass Filter and its instability as a result of the fast change in outside temperature is avoided. The PID Controller then fine tunes the flow control valve with an intention of reducing the error signal and thereby stabilizing the temperature of the reactor at the set point. Last, the reaction delay block represents the thermal mass and time constant which is involved between when the controller manipulates the heat supply of the system and the final response of the reactor temperature. In total, all the components are used in order to maintain the stability and effectiveness of the reactor’s temperature regulation even if encounters certain localized disturbances such as the changes of flow rate and delay. With the use of the PID controller, the system can change parameters constantly, which helps in keeping the temperature at the set value and enhances the reaction conditions as well as the stability of the process (Brune et al., 2022). This model is very useful in controlling processes occurring in industrial reactors in an efficient manner and avoiding issues like instability in temperatures or overshooting among others.
The plot in the MATLAB analysis presents the temperature response of the reactor system against time and offers information on the thermal behavior of the propane dehydrogenation process. Here, the time is plotted on the horizontal axis and the temperature shown on the vertical axis but this temperature has been scaled between 0 to 1 for convenience. There are some notable features in the temperature profile as follows; Firstly, there is a significant increase in temperature that is explained by the heat that is added in order to carry out the endothermic dehydrogenation process (Yonge et al., 2024). This is specifically important for raising the rate of increase to the required reaction temperature range. After having risen to this peak, the temperature drops down only to fluctuate to show that the system is responding to changes in heat and can be disrupted within the reactor.
The following series of the graph reveal fluctuations and abrupt rise and fall of temperature which indicates the presence of feedback systems which switch on and off the heat input to make steady the required temperature for the reactions. These might be due to the fact that the reaction is constantly changing, heat is constantly being taken in by the endothermic process, and the control system is providing more heat. Indeed, the temperature output curve has indicated how_successful operation of the control measures employed in the reactor design has been (He et al., 2024). It means that through careful control of temperature or possible fluctuations here, one is able to increase the conversion rate and selectivity as well as a good operational stability of the dehydrogenation reaction.
Simulink Model for Reactor Dynamics
The Simulink model starts with the Flow Rate input responsible for controlling the feed rate of the material into the reactor. This flow is regulated by a Flow Control Valve that depends on the control signal for regulating the input flow. The Reactor Dynamics block provides for determining the power level through the zero-order control block, which might contain a first-order transfer function mimicking the block-to-block heat changes with the reference to the input variations. After the temperature has been measured, it is compared to the desired temperature hence producing an Error Signal which will show the disparity between the two. A Lowpass filter also filters high-frequency noise from the temperature signal for some stability while the temperature fluctuates due to environmental conditions (Bere, 2022). The PID controller next adjusts the flow control valve in order to decrease the error signal and maintain the reactor temperature on the set point. The Reaction Delay is the mass and the time constant of the system where the controller alters the heating value and the reactor takes time to respond to the change in temperature. Maintaining the stabilities of the reactor’s temperature and ensuring effectiveness even in case of the irregularities like changes in the flow rate and delays is possible due to the involvement of this comprehensive model.
Analysis of the Error Signal Plot
The plot provided concerns the error signal which depicts the difference of the real temperature from the set temperature in the reactor system. The time is the x-axis and the y-axis defines the magnitude of the error signal normalized between 0 and 1 for this plot. The curves show large fluctuations in the output which represents the error signal that gives the difference between temperature set point and the actual temperature. These oscillations indicate that the control system is practicing to manage the heat supply to the reactor to achieve the right temperature needed for the dehydrogenation process (Cao et al., 2023). The oscillations of the pictured plot can be described as the control system’s oscillations due to changes of the reactor, for example in flow rate or due to the influence of the external temperature. Altogether, the error signal plot shows that the designed control system is capable of handling fluctuations within the temperature. What makes it possible is the steady regulation of the heat input to make sure that the reactor maintains the required operating temperature range that enhances on the conversion and selectivity to the dehydrogenation process (Chen et al., 2024). Such a control is especially necessary to keep the reactor’s system steady mining when dealing with large scale industries that require constant operation for longer periods.
These fluctuations are somewhat frequent and sharp which implies that the control system is actively trying to demonstrate stability. The high-frequency components, in the same manner, suggest that there is a presence of noise or high frequency changes that a control system has to counter. On the other hand, lower frequency LFO might indicate system dynamic at a lower rate or the lag in the feedback loop. The error signal can help the engineers in term of patterning and finding flaws in the control strategy of the system. For example, constant or very high oscillations may mean that the specific PID coefficients should be adjusted or the heat transfer in the reactor should be enhanced. Also, the error signal may be employed to check the ability of the control system in various operational conditions in making certain that the reactor performs with the same efficiency, despite of the circumstances (Nadjafi et al., 2023). In conclusion, the error signal plot helps in datum identification and acts as a means to know the state of the controlled system in order to fine-tune the control system for better output for effective operation of the reactor. Thus, to improve the performance of the reactor and productivity of the dehydrogenation process, the control system allows optimizing the error signal.
Conclusion
The preliminary design for PFR of propane dehydrogenation seems to be quite sturdy featuring a reactor capacity of 100 m³ and a reactor residency of 420 sec. The conversion is 95% and the production of propene is 580 mol/day. The following plots explain conversion as well as concentration profile and temperature distribution along the flow reactor. Despite that, there is room to perform extra optimization by making use of kinetic models and concrete measurements of industrial reactor reality. The task of designing a PFR reactor for dehydrogenation of propane to propene is complex and involves several aspects such as reaction kinetics, geometry of the reactor, mass and energy balances, and control system. It is therefore important to investigate reaction kinetics to arrive at the best diagnostic and strategies for catalyst optimization. The disposal of exerted reactor also implies an improved flow distribution which reduces the pressure drop thus increasing the overall efficiency. Not only does it increase the efficiency of the thermal energy recovery, but rather there is minimal energy loss since heat produced during the reaction is recycling in the process. Subsequent work on the corrosion catalysts should be directed towards enhancing the activity, selectivity and stability of the catalysts. Further, second, reactor engineering and the connection of the process with renewable energy sources can positively contribute to the economy and constant delivery of the process. On this regard, the following aspects in realization of the dehydrogenation process of the propane can enhance the formation of propene that is essential for the preparation of petrochemical products.
Struggling with tight deadlines or complex university assignments? Native Assignment Help provides reliable assignment help UK services, supporting students with structured writing, research, and editing that meet academic standards and submission requirements.
Reference List
Journals
Gambo, Y., Adamu, S., Abdulrasheed, A.A., Lucky, R.A., Ba-Shammakh, M.S. and Hossain, M.M., 2021. Catalyst design and tuning for oxidative dehydrogenation of propane–A review. Applied Catalysis A: General, 609, p.117914.
Oyegoke, T., Dabai, F.N., Waziri, S.M., Uzairu, A. and Jibril, B.Y., 2024. Computational study of propene selectivity and yield in the dehydrogenation of propane via process simulation approach. Physical Sciences Reviews, 9(2), pp.1049-1063.
Carter, J.H., Bere, T., Pitchers, J.R., Hewes, D.G., Vandegehuchte, B.D., Kiely, C.J., Taylor, S.H. and Hutchings, G.J., 2021. Direct and oxidative dehydrogenation of propane: from catalyst design to industrial application. Green Chemistry, 23(24), pp.9747-9799.
Martino, M., Meloni, E., Festa, G. and Palma, V., 2021. Propylene synthesis: Recent advances in the use of Pt-based catalysts for propane dehydrogenation reaction. Catalysts, 11(9), p.1070.
Yang, F., Zhang, J., Shi, Z., Chen, J., Wang, G., He, J., Zhao, J., Zhuo, R. and Wang, R., 2022. Advanced design and development of catalysts in propane dehydrogenation. Nanoscale, 14(28), pp.9963-9988.
Sun, M.L., Hu, Z.P., Wang, H.Y., Suo, Y.J. and Yuan, Z.Y., 2023. Design strategies of stable catalysts for propane dehydrogenation to propylene. ACS catalysis, 13(7), pp.4719-4741.
Brune, A., Geschke, A., Seidel-Morgenstern, A. and Hamel, C., 2022. Modeling and simulation of catalyst deactivation and regeneration cycles for propane dehydrogenation-comparison of different modeling approaches. Chemical Engineering and Processing-Process Intensification, 180, p.108689.
Matthies, J.H., Dittmann, D., Dyballa, M., Tuttlies, U. and Nieken, U., 2024. Investigation of Aging Mechanism of Pt‐ZSM‐5 Catalysts for Non‐Oxidative Propane Dehydrogenation. Chemie Ingenieur Technik, 96(6), pp.850-863.
Kumar, P. and Srivastava, V.C., 2023. Elucidation of catalytic propane dehydrogenation using theoretical and experimental approaches: Advances and outlook. Energy & Fuels, 37(23), pp.18369-18394.
Motagamwala, A.H., Almallahi, R., Wortman, J., Igenegbai, V.O. and Linic, S., 2021. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science, 373(6551), pp.217-222.
Ingale, P., Knemeyer, K., Preikschas, P., Ye, M., Geske, M., d'Alnoncourt, R.N., Thomas, A. and Rosowski, F., 2021. Design of PtZn nanoalloy catalysts for propane dehydrogenation through interface tailoring via atomic layer deposition. Catalysis Science & Technology, 11(2), pp.484-493.
Wang, G.D., Jiang, J.W., Sui, Z.J., Zhu, Y.A. and Zhou, X.G., 2022. Kinetic Promotion Effect of Hydrogen and Dimethyl Disulfide Addition on Propane Dehydrogenation over the Pt–Sn–K/Al2O3 Catalyst. ACS omega, 7(35), pp.30773-30781.
Huš, M., Kopač, D., Bajec, D. and Likozar, B., 2021. Effect of surface oxidation on oxidative propane dehydrogenation over chromia: an ab initio multiscale kinetic study. ACS catalysis, 11(17), pp.11233-11247.
Hannagan, R.T., Giannakakis, G., Réocreux, R., Schumann, J., Finzel, J., Wang, Y., Michaelides, A., Deshlahra, P., Christopher, P., Flytzani-Stephanopoulos, M. and Stamatakis, M., 2021. First-principles design of a single-atom–alloy propane dehydrogenation catalyst. Science, 372(6549), pp.1444-1447.
Zhang, Y., Yu, Y., Dai, Y., Zhang, Y., Liu, Q., Xiong, D., Bao, L., Wu, Q., Shi, D., Chen, K. and Li, Y., 2023. Regulating the C–H bond activation pathway over ZrO2 via doping engineering for propane dehydrogenation. ACS Catalysis, 13(10), pp.6893-6904.
Yonge, A., Gusmão, G.S., Fushimi, R. and Medford, A.J., 2024. Model-Based Design of Experiments for Temporal Analysis of Products (TAP): A Simulated Case Study in Oxidative Propane Dehydrogenation. Industrial & Engineering Chemistry Research, 63(11), pp.4756-4770.
He, Z., Yang, J. and Liu, L., 2024. Design of Supported Metal Catalysts and Systems for Propane Dehydrogenation. JACS Au, 4(11), pp.4084-4109.
Walter, J.P., Brune, A., Seidel-Morgenstern, A. and Hamel, C., 2021. Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor. Catalysts, 11(9), p.1056.
ZHANG, X.Y., Hai, W.A.N., Yuan, G.A.O., BAO, J.Q. and ZHANG, H.J., 2022. Effect of propylene in feedstock on the coking behavior of PtSnK/Al2O3 catalyst of propane dehydrogenation. Journal of Fuel Chemistry and Technology, 50(7), pp.841-849.
Go Through the Best and FREE Samples Written by Our Academic Experts!
Native Assignment Help. (2026). Retrieved from:
https://www.nativeassignmenthelp.co.uk/reactor-design-for-propane-dehydrogenation-46894
Native Assignment Help, (2026),
https://www.nativeassignmenthelp.co.uk/reactor-design-for-propane-dehydrogenation-46894
Native Assignment Help (2026) [Online]. Retrieved from:
https://www.nativeassignmenthelp.co.uk/reactor-design-for-propane-dehydrogenation-46894
Native Assignment Help. (Native Assignment Help, 2026)
https://www.nativeassignmenthelp.co.uk/reactor-design-for-propane-dehydrogenation-46894
- FreeDownload - 39 TimesCyber Security Application Portfolio Assignment Sample
Introduction - Cyber Security Application Portfolio Cyber security is a...View or download
- FreeDownload - 42 TimesBusiness Development And Value Creation Business Plan Assignment Example
1. Introduction - Business Development And Value Creation Business Plan A...View or download
- FreeDownload - 40 TimesDebate The Use Of Mass Surveillance Technology By Governments Assignment
DEBATE THE USE OF MASS SURVEILLANCE TECHNOLOGY BY GOVERNMENTS...View or download
- FreeDownload - 38 TimesBUS7049: Cross-Cultural Management Assignment Sample
BUS7049: Cross-Cultural Management Assignment Sample Review of Journal...View or download
- FreeDownload - 45 TimesDigital Innovation in construction for passivhaus student accommodation
Digital Innovations In The Design Stage For Passivhaus Construction In UWE...View or download
- FreeDownload - 45 TimesFDY3005 Becoming an Effective Leader
Description of your business idea · Describe your business...View or download
-
100% Confidential
Your personal details and order information are kept completely private with our strict confidentiality policy.
-
On-Time Delivery
Receive your assignment exactly within the promised deadline—no delays, ever.
-
Native British Writers
Get your work crafted by highly-skilled native UK writers with strong academic expertise.
-
A+ Quality Assignments
We deliver top-notch, well-researched, and perfectly structured assignments to help you secure the highest grades.